R&D
- Home
- R&D | Research and Development
- Clinical Research
- Basic and Translational Research(TR)
- National Projects
Clinical trials for Commercialization
| Product Name | Indication | Stages of Clinical Trials | Clinical Test Institute | Number of Test Targets | Date of Approval by Ministry of Foods and Drug Safety of Korea |
|---|---|---|---|---|---|
| Hearticellgram®_AMI | Acute myocardial infarction | [ Approval completed ] Additional phase 3 in progress |
9 institutes in addition to Severance Hospital | 135 persons | Jul. 7, 2011 |
| Cellgram_LC | Cirrhosis | Phase 3 | 8 Institutes in addition to Wonju Christian Hospital | 200 persons | Dec. 2, 2020 |
| Cellgram_ED | Impotence | Phase 2 | Seoul Asan Hospital | 54 persons | Jun. 12, 2020 |
| Cellgram_CKD | Chronic Kidney Disease | Phase 1 | Seoul Asan Hospital | 10 persons | Jul. 25, 2021 |
Clinical trials of Researcher
| Titles of Clinical Tests | Date of Approval by Ministry of Food and Drug Safety | Clinical Stage | Clinical Test Institutes |
|---|---|---|---|
| A researcher-led clinical test to compare and evaluate safety and effectiveness for injection of autologous marrow-derived mesenchymal stem cell drug in patients with chronic glomerular nephritis | Aug. 19, 2014 | Researcher Clinical test |
Kyunghee University Hospital National heatlth Insurance Service Ilsan Hospital |
| A researcher-led clinical study to compare and evaluate safety and effectiveness of intravenous injection or coronary artery injection of autologous marrow-derived mesenchymal stem cell drug in patients with acute cardiac infarction | May 30, 2014 | Researcher Clinical test |
Hyewon Medical Corporation Seijong Hospital |
| A pilot clinical test to explore safety and effectiveness of transplantation of autologous marrow-derived mesenchymal stem cell in patients with auditory nerve disease or sensorineural deafness | Jan. 17, 2014 | Researcher Clinical test |
Educational Foundation Catholic University Seoul St. Mary Hospital |
| A pilot clinical test for safety and treatment effects of mesenchymal stem cell in patient with critical respiratory failure | July 18, 2013 | Researcher Clinical test |
Asan Foundation Corporation Seoul Asan Hospital |
| A clinical test of cell transplantation and treatment using autologous mesenchymal stem cell cultured from autologous serum in patients with cerebral infarction | Oct. 15, 2012 | Researcher Clinical test |
Samsung Seoul Hospital |
Technology development for culture and division of mesenchymal stem cell
- Technology development that can effectively differentiate mesenchymal stem cell stably and in high purity from marrow, cord blood and fat tissue.
- Technology development (culture medium) that can multiply in great numbers while retaining the feature of stem cell (multipotent).
- Developing culture medium(no-serum medium) excluding animal-drive elements such as FBS for establishment of safety of mesenchymal stem cell.
Differentiation related technology development
- Explore genes or proteins related to induction or inhibition of differentiation of mesenchymal stem cell.
- Clarify the signaling system related to differentiating regulation.
- Improve and optimize technology to induce differentiation of specific tissue cell(nerve cell, cardiac cell, pulmonary alveoli cell).
- Explore methods of differentiation into new cells.
- Identify new marker related to differentiated and un differentiated mesenchymal stem cells.
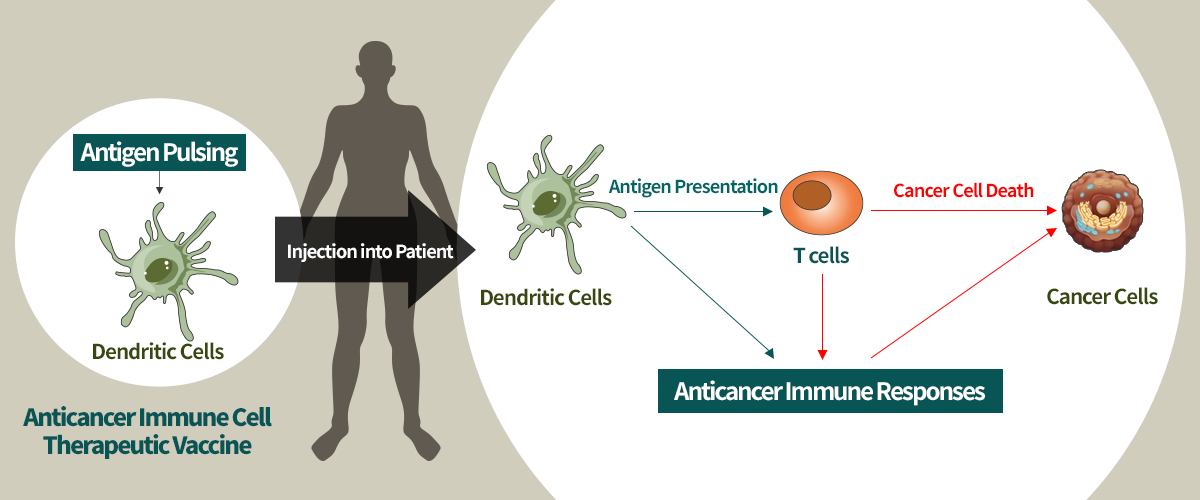
Research and Develop Anti-cancer Immune Vaccine for Treatment
- Develop production of dendritic cell drug technology with much better quality and quantity compared to existing ones since culture source of dendritic cells for treatment can be proliferated and differentiated
- Develop production of dendritic cell drug technology with much better quality and quantity compared to existing ones since culture source of dendritic cells for treatment can be proliferated and differentiated
- Develop clinical protocol for the most effective and strongest anticancer immuneity treatment through combined administration of existing anticancer drug or natural material and gene medicine
- Established cell culture condition for optimal treatment and develop commercialization technology through dendritic cell differentiation signal study
Secure Quality Control Technology
- stablish quality control law suitable for standards necessary to secure consistency of quality and safety of stem cell medicine (Regulation on Review and Authorization of Biological Products, Notice of Ministry of Foods and Drug Safety, No. 2015-41)
- Produce the best stem cell medicine by applying it to final complete medicine and manufacturing process
Develop Gene Carrier System
- Develop mesenchymal stem cell monitoring technology for the transplantation
- Establish and improve specific gene expression and inhibition technology
- Study on availability of mesenchymal stem cell for gene therapy
| Bone marrow-derived mesenchymal stem cells-development of anti-cancer immune cell therapy |  |
|
Project name : Development of anticancer immune cell therapy using myeloid stem cell-derived next-generation dendritic cells Period : 2016.11.08 ~ 2021.07.31 |
|
| Marrow-derived mesenchymal stem cell - develop auto culture system for mesenchymal stem cell drug |  |
Project name : development of unmanned automated cell drug production system Period : 2014.11.01 ~ 2017.10.31 |
|
| Marrow-derived mesenchymal stem cell - development of alcohol cirrhosis drug |  |
Project name : Phase 2 commercialization clinical trial to evaluate safety and effectiveness of autologous mesenchymal stem cell transplantation treatment in patient with alcoholic cirrhosis. Period : 2014.10.01 ~ 2016.09.30 |
|
| Marrow-derived mesenchymal stem cell - development of stem cell aging measurement system |  |
Project name: Development of Oligonucleotide-linked Immunosorbent Assay (OLISA)-based non-labeled and real-time mesenchymal stem cell aging measurement system. Period : 2013.06.01 ~ 2015.05.30 |
|
| Marrow-derived mesenchymal stem cell - development of cirrhosis stem cell drug for USA clinical test |  |
Project name : Consulting for clinical advancement and FDA Pre-IND Meeting for approval of stem cell drug for hepatic insufficiency(alcoholic cirrhosis) at clinical test of USA. Period : 2013.06.01 ~ 2015.11.30 |
|
| Marrow-derived mesenchymal stem cell - development of impotence drug |  |
Project name : Non-clinical study for marrow-derived mesenchymal stem cell drug for treatment of incurable impotence. Period : 2012.12.26~2014.11.25 |
|
| Marrow-derived mesenchymal stem cell - development of critical lower |  |
Project name : limb ischemia drug Non-clinical trial research for development of stem cell drug for critical lower limb ischemia. Period : 2012.12.26 ~ 2014.11.25 |